20+ avogadro's number calculator
The Avogadros constant or Avogadros number is the number of units in one mole of any substance. M Average atomic mass D.

Calculate The Value Of Avogadro S Number From The Following Data Density Of Nacl Youtube
Depending on the nature of the reaction and the.

. But remember that M is. To use this online calculator for Mass of Atom of Element using Avogadros Number enter Gram Atomic Mass GAM and hit the calculate button. Volume or number of moles and select which value you want to find out eg.
Online calculator which helps you calculate the Avogadros number. This Avogadros law calculator determines the initial and final volumes and quantities in moles of a system with an ideal gas if its pressure and temperature. The Avogadro law states that at constant pressure and temperature two volumes of two ideal gases have the same molar volume.
Avogadros number is nothing but the amount of particles found in 1 mole of a substance. Practice Problems using Avogadros Number. Through experiments the value is found to be 6022 x 10 23 ie.
So Avogadros number consists formally of the number of atoms that make up such 12 g of carbon 12. Estimation of Calcium by Permanganometry Calculator. Integers decimal or the E-notation form of scientific notation ie.
Combined Gas Law Calculation. Enter known values eg. This tool will calculate any parameter from the equation defined by Avogadros law which includes the V 1 gas volume n 1 amount of gas V 2 gas volume and n 2 amount of gas.
Calculations related to Avogadros law. To use this online calculator for Final Number of Moles of Gas by Avogadros Law enter Final volume of gas V 2 Initial volume of gas V 1 Initial moles of gas n 1 and hit the calculate. 6022140857 x 1023 is its value.
The number 602 10 23 is known as Avogadros number N A and it represents the number of particles contained in 12 g of Carbon - 12. This relation is then used to convert a number of H 2 O molecules to grams by the ratio. In other words 1 mol of carbon-12 has a mass of 12.
The calculator below can compute very large numbers. Calculation of One Mole How to calculate Avogadro Number This video lecture will help you to understand the quantity of one mole you can understand how t. Avogadros number in chemistry and physics is the number of particles atoms or molecules contained in 1 mole of a.
One mole of H 2 O is 6022 x 10 23 molecules of H 2 O Avogadros number. You see that you are given the 1 volume 2 density and 3 the Molar Mass M. In other words at constant pressure and temperature two.
You can use these two formulas to calculate Avogadros number. And its unit is the mole and its derivatives kmol mmol lb-mole etc. Amadeo Avagadro proposed in 1811 that equal volumes of gas contains an equal number of atoms or molecules if the two volumes are held at the same temperature and.
Here is how the Mass of Atom of Element.

Avogadro S Law Calculator Thermodynamics Heat Online Unit Converters

Calculating Moles Using Avogadro S Number Youtube

Key Strategy For The Rational Incorporation Of Long Lived Nir Emissive Cr Iii Chromophores Into Polymetallic Architectures Inorganic Chemistry

Pdf Visualizing The Avogadro Number
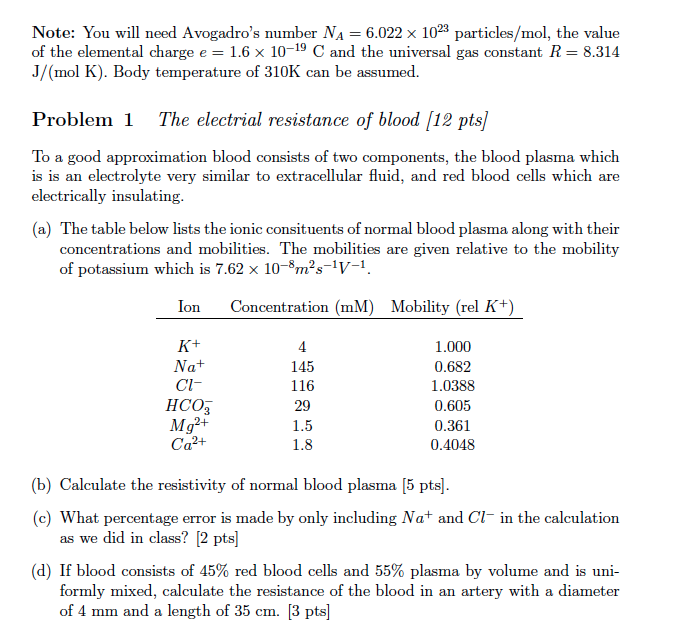
You Will Need Avogadro S Number Na 6 022 X 1023 Chegg Com
Chemteam Using Avogadro S Number In Calculations

Calculating Moles Using Avogadro S Number Youtube
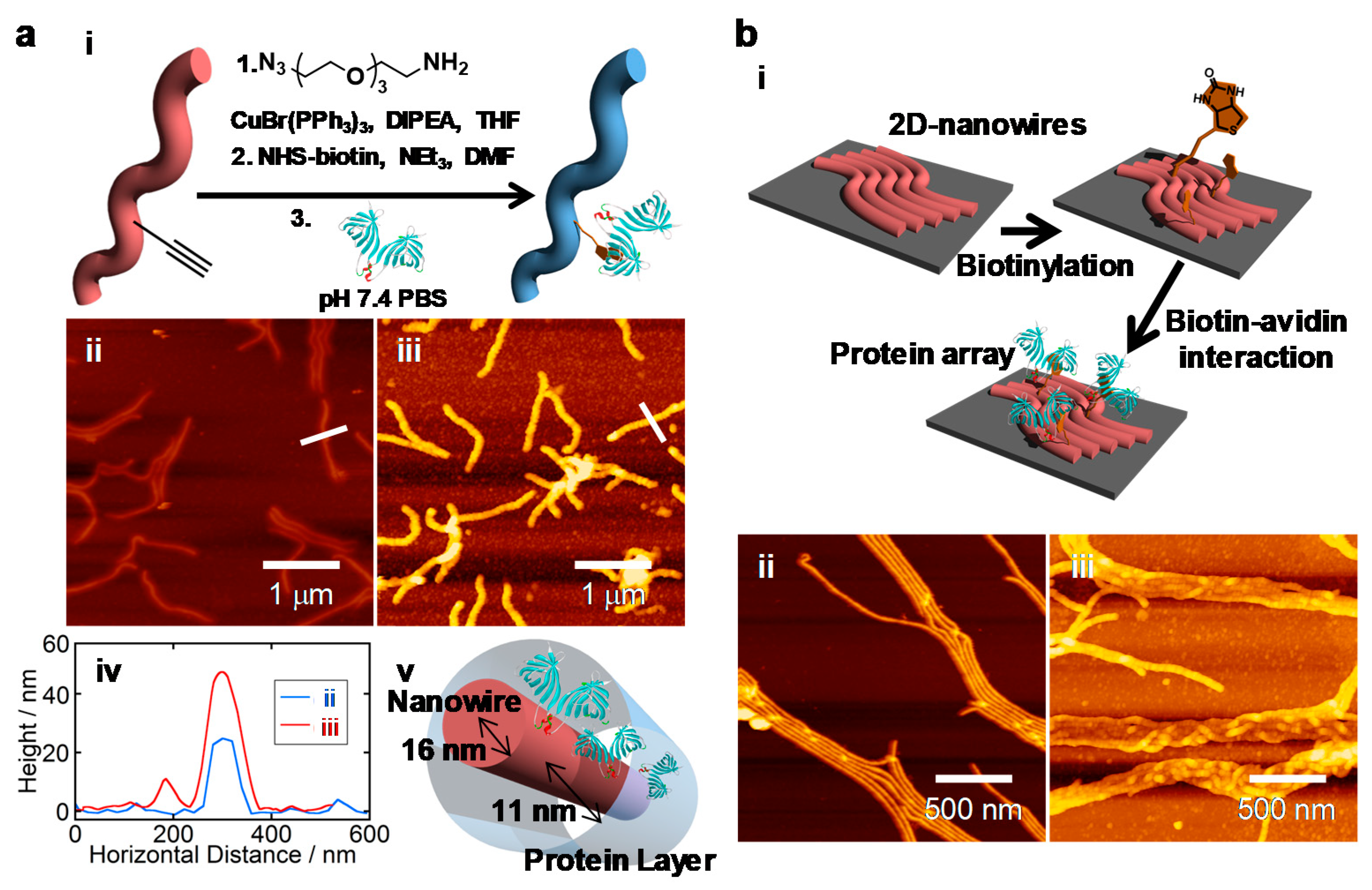
Qubs Free Full Text Interactions Of Single Particle With Organic Matters A Facile Bottom Up Approach To Low Dimensional Nanostructures Html

Sandra Tiernan Tiernansandra Twitter

How To Use Your Scientific Calculator Youtube

Molar Mass Teaching Resources Teachers Pay Teachers
What Is A Gram Mole Quora

Pdf Review Of Particle Physics Ed Blucher Academia Edu

Pdf Mole Concept And Problems Solving Approaches In Life Sciences
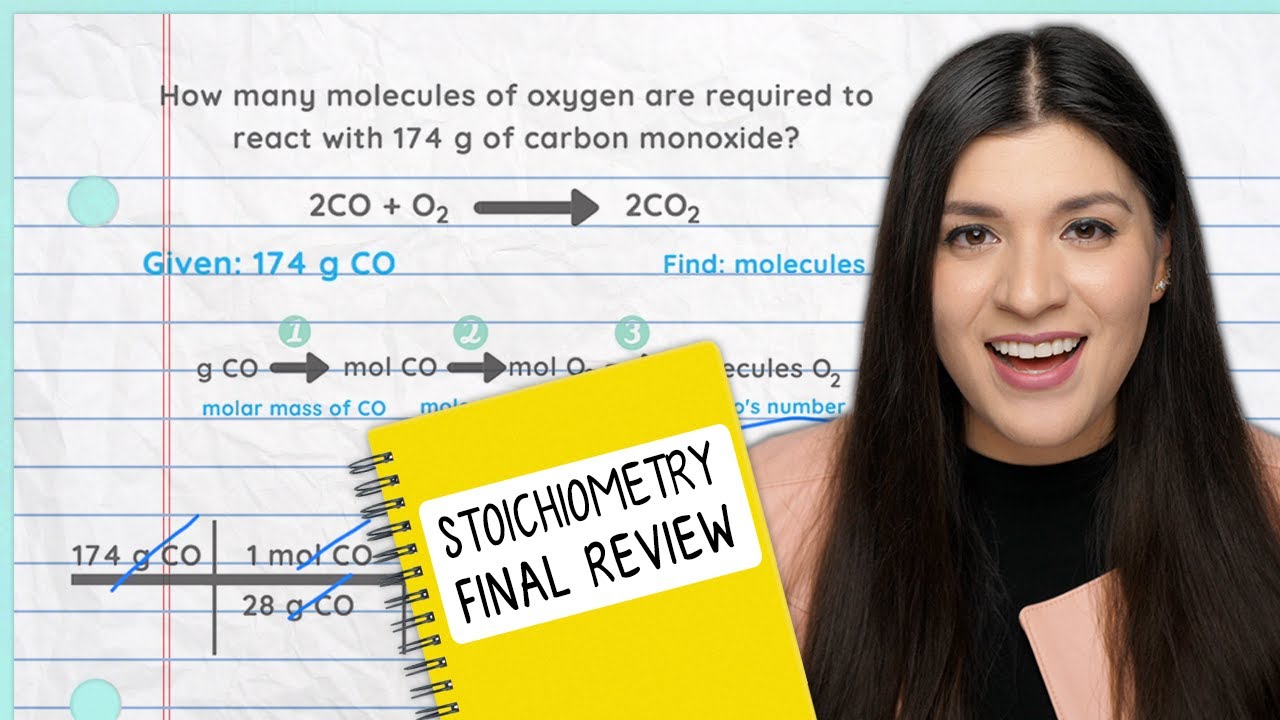
Using Avogadro S Number How To Pass Chemistry Youtube

What Is Avogadro S Number Howstuffworks

Computation In The Human Cerebral Cortex Uses Less Than 0 2 Watts Yet This Great Expense Is Optimal When Considering Communication Costs Biorxiv